Medcura was founded to capitalize on revolutionary biochemistry discoveries that can transform highly abundant natural materials into advanced wound treatment and hemostatic (bleeding management) products. Medcura’s growing line of products is designed to obtain hemostasis using hydrophobically modified chitosan, a biomaterial which also has antimicrobial properties. These easy-to-apply, stable, and uniquely versatile product formats may include, but is not limited to gels, bandages, foams, and more. The primary advantage of our approach is the formation of a mucoadhesive barrier that keeps blood where it is supposed to be (i.e., inside the patient) and other pathogens outside the site. Medcura’s platform of products is being engineered to be used across a broad spectrum of clinical settings, which could include surgical bleeds, routine lacerations, nosebleeds, and traumatic injuries.
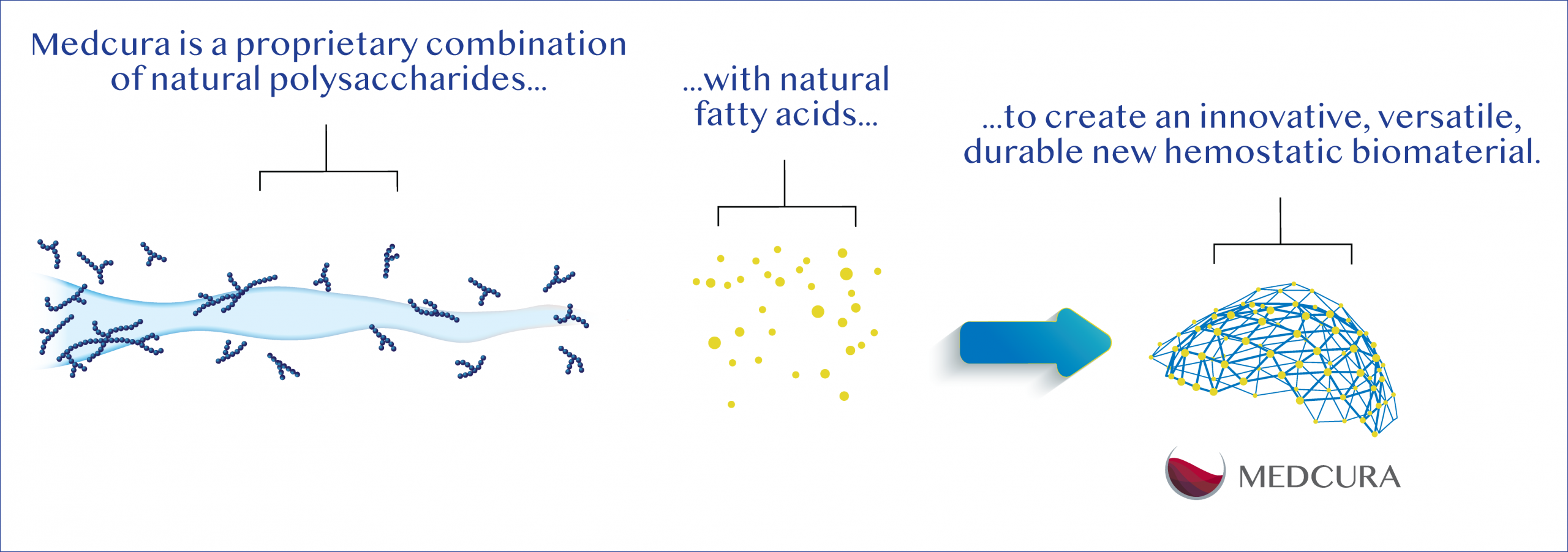
Medcura relies on a combination of safe and naturally abundant ingredients as the foundations for all its products, which also allows for the creation of disruptive cost-effective product and scalable manufacturing. Products are created by applying proprietary modifications to native chitosan mixed with plant-based fatty acids, resulting in compounds that are mucoadhesive, have antimicrobial properties, are biocompatible, and stable. When applied to a wound, these hydrophobically-modified compounds act as a viscoelastic matrix that rapidly self-assembles into a robust mechanical barrier designed to stop bleeding independent of the body’s clotting cascade.
*K143466 Rx: IIndicated for use, under the direction of a healthcare professional, in the management of bleeding wounds such as vascular access sites and percutaneous catheters or tubes. Not yet commercially available.
K172010 Rx: Indicated for Management for the management of moderately to heavily exuding chronic wounds and acute wounds. Under medical supervision, may be used for management of pressure sores, diabetic ulcers, leg ulcers, donor sites and graft sites, surgical wounds, skin abrasions and lacerations, 1st and 2nd degree burns, and trauma wounds.
OTC: Indicated for management of minor cuts, minor scalds and 1st-degree burns, abrasions, and lacerations. Not yet commercially available.
K180152 OTC/ K182811 OTC/ K192667 OTC: Indicated for the local management of bleeding wounds such as minor cuts, minor lacerations, and minor abrasions (antibacterial & antibacterial, under non-sterile processing).
Additional products under development, not yet cleared or approved and not yet commercially available.